Dr. Fazio has a background in mechanical engineering, and as part of the UAB Ocular Biomechanics and Mechanobiology group his work primarily focuses on developing advanced image processing algorithms and custom optical devices in order to investigate the biomechanics and morphology of ocular tissue in glaucoma and myopia.
At the award ceremony, Dr. Fazio presented a riveting lecture entitled “In-vivo Quantification of Biomechanics and Morphometry across Ocular Disease”, which illustrated how custom methods of image analysis combined with unique imaging modalities are applied to human and animal models. These methods set the stage for novel imaging tools that may aid in the diagnosis of various ocular diseases.
With the annual Xtreme Research Award, Heidelberg Engineering intends to honor cutting-edge research for the advancement of ophthalmic care. This year, Dr. Fazio’s work was recognized for its contribution to identifying novel imaging biomarkers that take into account the dynamic biomechanical response of ocular tissues to intraocular pressure. This response is thought to be involved in the pathogenesis of glaucoma and myopia.
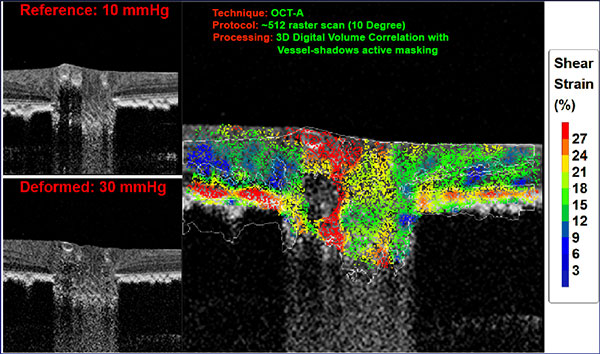
Although still in its early stages, Dr. Fazio’s research aims to develop imaging methods that can enhance the current OCT-based diagnostic parameters and offer customized analyses that may be predictive of future damage. One such method is the use of a simple and quick image scanning protocol that can assess each eye’s unique mechanical response by the optic nerve head to IOP changes. The evaluation of such changes offers shear strain values that may be indicative of the eye’s susceptibility to damage due to diurnal IOP variations and of future visual field loss. If this hypothesis is proven true, customized measurements of characteristics such as retinal nerve fiber layer, lamina cribrosa shifts, and peripapillary sclera changes could lead to improved glaucomatous OCT parameters and diagnostically sensitive predictive factors.
“The imaging capabilities achieved by the SPECTRALIS OCT allowed us to visualize and quantify the complex biomechanical behavior of ocular tissue in-vivo and in real-time. This quantification has never been done before. Quantitative eye-specific analyses that were only achievable in a research environment are soon going to be transferred to daily clinical practice. I believe that with the widespread use of new OCT technologies, we’ll soon witness a radical shift on how we diagnose and treat debilitating ocular diseases”, said Dr. Fazio.
“Moving forward, we would like to provide a diagnostic imaging platform to offer a suite of diagnostic parameters that extend beyond static measures and are customized to each eye’s unique anatomy. We are excited about Dr. Fazio’s research project and hope that such tools will enable clinicians to better predict their diagnostic and treatment outcomes, offering the opportunity to make more informed decisions with higher clinical confidence,” said Ali Tafreshi, Director of Clinical Research, Heidelberg Engineering.
For that purpose, Heidelberg Engineering’s Research and Development team supports Dr. Fazio and the UAB Ocular Biomechanics and Mechanobiology group as well as other leading scientists eager to enhance OCT-based diagnostic parameters. Heidelberg Engineering aims to expand its collaborative efforts with a growing number of researchers around the world to offer novel OCT-based methods geared towards individualized assessment of various ocular pathologies such as glaucoma.